
These investigations often moved from table top experiments within the original Cavendish Laboratory, to bigger and bigger machines. By the 1920s, many scientists were studying electrons as part of the new discipline of quantum physics. The Cavendish Laboratory became an international scientific centre, attracting graduate students from across Britain and the world. This discovery was just one of the exciting developments in Cambridge physics around the turn of the nineteenth century, as physicists turned to probing the very state of matter. In this way, the announcement of the discovery was the icing on top of a giant cake of collaborative and cumulative work! The science behind this announcement began long before April 1897, and Thomson had been using a variety of instruments to investigate phenomena such as ‘cathode rays’. However, Thomson recognised the importance of this phenomenon as a constituent of matter, and identified a way in which the electron could be manipulated and experimented upon. This discovery led to the ‘plum pudding’ model of the atom which viewed the fundamental building blocks of matter as negatively charged corpuscles scattered throughout a cloud of positive charge. He found that their mass to charge ratio was 1000 times smaller than the hydrogen ion – and called these new particles ‘corpuscles’. It was known that these rays could be deflected by a magnetic field, and Thomson used this property to investigate whether these rays were negatively charged particles.

These rays caused fluorescence when they hit the far wall of the tube.

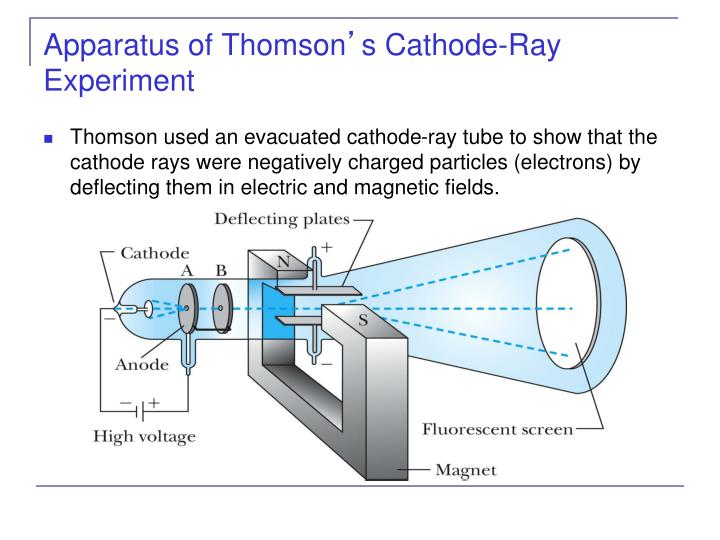
He investigated the conduction of electricity through gases – work for which he would win a Nobel Prize in 1906.įor the 1897 experiment, Thomson used an electric discharge tube which produced ‘cathode rays’ at low pressures.
The rays produced in a cathode tube are free#
In the Cavendish Laboratory, then located on Free School Lane, Thompson conducted experiments as Director of the Laboratory. Thomson announced his discovery of negatively charged constituents of the atom, which we now call electrons. Another one of these spots is located just outside the SPS Library on Free School Lane: the laboratory where the electron was discovered.ġ22 years ago, on the 30th of April 1897, J. Perhaps the pub where Watson and Crick ‘discovered’ DNA or the Mathematical Bridge, supposedly ‘designed by Isaac Newton’. The advantage of the hot cathode was that it had a much longer working life, and its output of X-rays could be controlled by adjustments made to the heating element current and to the potential difference between the cathode and the anticathode.Whenever you walk through central Cambridge, you will encounter tour groups gathering at key spots throughout the city centre. On hitting the anticathode they caused the emission of X-rays just as they did in the gas tube. As a consequence the electrons that it produced due to the high temperature passed as a stream of cathode rays to the positively charged anticathode at the other end of the tube. As well as carrying the heating current, however, the spiral was also raised to a high negative potential. In this the cathode was a spiral wire heated to incandescence by a small electric current as in an electric-light bulb. Furthermore, the intensity of the X-ray beams which they produced was so low that inconveniently long exposures were needed to produce a good X-ray picture, or radiograph. As such it protected the tube walls from the damaging effects of cathode-ray bombardment.Īlthough these early gas tubes worked, they were never very satisfactory for they deteriorated rapidly with use. Each tube contained an anode and a cathode, but, in addition, there was a third component known as an anticathode, which was placed immediately opposite the cathode so that all the cathode rays impinged directly on it instead of on the wall. In those early days the tubes that were used were known as gas tubes, for their successful operation depended on the small quantity of air that was intentionally left in the tubes when they were evacuated. To a metal anode, where their impacts produce X-rays.įollowing Wilhem Röntgen's discovery of X-rays in 1895, applications of the new rays were so immediately apparent, particularly in diagnostic medicine, that people all over the world began to build and use X-ray equipment. An X-ray tube is a vacuum tube containing electrodes that


 0 kommentar(er)
0 kommentar(er)
